
- •Preface
- •Acknowledgments
- •Basic Physics
- •Transducers and Resolution
- •Doppler Physics
- •Artifacts
- •Summary
- •Introduction
- •Patient Preparation
- •Patient Positioning
- •Transducer Selection
- •Two-Dimensional Images
- •Two-Dimensional Imaging Controls
- •Introduction
- •M-Mode Echocardiography
- •Color-Flow Doppler
- •Spectral Doppler
- •Tissue Doppler Imaging
- •Measurement and Assessment of Two-Dimensional Images
- •Measurement and Assessment of M-Mode Images
- •Measurement and Assessment of Spectral Doppler Flow
- •Measurement and Assessment of Tissue Doppler Imaging
- •Evaluation of Color-Flow Doppler
- •Evaluation of Ventricular Function
- •Mitral Regurgitation
- •Aortic Regurgitation
- •Tricuspid Regurgitation
- •Pulmonary Regurgitation
- •Endocarditis
- •Pulmonary Hypertension
- •Systemic Hypertension
- •Hypertrophic Cardiomyopathy
- •Dynamic Right Ventricular Outflow Obstruction
- •Moderator Bands
- •Dilated Cardiomyopathy
- •Right Ventricular Cardiomyopathy
- •Restrictive Cardiomyopathy
- •Endocardial Fibroelastosis
- •Arrhythmogenic Right Ventricular Cardiomyopathy
- •Myocardial Infarction
- •Myocardial Contusions
- •Pericardial Effusion
- •Neoplasia as a Cause of Pericardial Effusion
- •Pericardial Disease
- •Abscesses
- •Pericardial Cysts
- •Thrombus
- •Ventricular Septal Defect
- •Patent Ductus Arteriosus
- •Aorticopulmonary Window
- •Right to Left Shunting PDA
- •Atrial Septal Defects
- •Endocardial Cushion Defects
- •Bubble Studies
- •Atrioventricular Valve Dysplasia
- •Outflow Obstructions
- •Inflow Obstructions
- •Tetralogy of Fallot
- •APPENDIX ONE Bovine
- •APPENDIX TWO Canine
- •APPENDIX THREE Equine
- •APPENDIX FOUR Feline
- •APPENDIX FIVE Miscellaneous Species
- •Index

Differentiation from Other Disease
Differentiation of RCM from other heart disease is often difficult. Hypertrophic cardiomyopathy and constrictive pericarditis are the two most common differentials (186). Constrictive pericarditis is associated with respiratory variation in peak mitral and tricuspid inflow velocities, pulmonary venous flow, and isovolumic relaxation times (Figure 8.32). RCM does not show this respiratory variation (123,185).
Hypertrophic cardiomyopathy may show the restrictive inflow pattern associated with RCM. In man when restrictive physiology is present in the absence of outflow obstruction and only mild to moderate myocardial hypertrophy as well as a disproportionately large atrium compared to ventricular changes are present, then the diagnosis of RCM is made versus HCM (123). It is a challenge to differentiate, but the therapeutic approach is often the same and so the distinction is often not critical (44).
Endocardial Fibroelastosis
Endocardial fibrosis in the dog has been described, and features include a dilated left ventricular chamber with decreased wall and septal motion, depressed fractional shortening, dilated left atrium and auricle, and abnormal mitral valve morphology and motion. Endocardial fibrosis was not described as an echocardiographic finding since these studies were obtained with M-modes, but necropsy findings showed extensive endocardial and myocardial fibrosis with variable areas of hypertrophy (180). The mitral apparatus is involved in this disease process with thickening of the leaflets and chordae. This disease is differentiated from degenerative mitral valve disease by the excellent systolic function present in most hearts with mitral insufficiency secondary to endocardiosis (180). This disease is only reported in young dogs, and its status as an acquired defect is debatable.
Arrhythmogenic Right Ventricular Cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy is an infiltrative disease of the right ventricular myocardium. Fatty and fibrous infiltration leads to ventricular and supraventricular arrhythmias that often result in sudden death (171). The infiltrative process may involve the left ventricular chamber in some cases (171).
This disease is reported in cats with echocardiographic manifestations that include pronounced right atrial and ventricular dilation, paradoxical septal motion, abnormal trabeculation of the right ventricular wall, and aneurysmal dilation of the right ventricular wall with akinetic and dyskinetic muscle (171). The left side of the heart is essentially normal. Abnormalities of the left side when present include atrial dilation and abnormal fractional shortening in the presence of paradoxical septal motion. The left atrial dilation ranges from mild to severe in these cats (171).
Arrhythmogenic Cardiomyopathy
Usually has no echocardiographic abnormalities
Most affected dogs with ARVC have no detectable echocardiographic abnormalities. Some Boxers, with arrhythmia, have left ventricular dysfunction, but it is not a common occurrence. Careful evaluation of the right side of the heart may show some enlargement and dysfunction, but this is a challenge (186).

Myocardial Infarction
Myocardial infarction does not occur frequently in animals and when it does is typically secondary to other cardiac disease such as aortic stenosis, cardiomyopathy, neoplasia, or endocarditis (187). Atherosclerosis occurs in dogs, especially in hypothyroid animals (188). The features of acute myocardial ischemia on echocardiograms include reduced wall or septal systolic thickening, dyskinetic wall motion, and systolic thinning as opposed to thickening of the wall or septum (189– 195). The infracted myocardium will become thinner and appear echodense as the affected areas become fibrotic. This is also reported in a horse (Figures 7.50, 7.51, 7.52, 7.53, 7.54) (196–198).
Figure 7.50 Thin myocardium with a small aneurysmal dilation in the apex of the right ventricle is indicative of infarcted muscle (arrow). This dog also has a small pericardial effusion. RV = right ventricle, LV = left ventricle, RA = right atrium, LA = left atrium, plane = modified apical four chamber, PE = pericardial effusion.
Figure 7.51 An area of fibrosis and thinning in this ventricular septum is the site of an old myocardial infarction (arrow). RV = right ventricle, LV = left ventricle, VS = ventricular septum, plane = right parasternal four chamber, species = dog.

Figure 7.52 A dense echogenic area within the myocardium of this horse was found to be an area of infarcted myocardium at necropsy. RV = right ventricle, LV = left ventricle, plane = right parasternal transverse left ventricle.
Figure 7.53 This thin free wall with no systolic thickening is consistent with an old myocardial infarction on this M-mode image of the left ventricle. RV = right ventricle, LV = left ventricle, PE = pericardial effusion, species = feline.

Figure 7.54 The (A) two-dimensional and (B) M-mode images of this cat’s heart show a thin left ventricular wall with poor function. Septal function is normal. RV = right ventricle, LV = left ventricle, AO = aorta, LA = left atrium, LVW = left ventricular wall, plane = right parasternal left ventricular outflow view.

When an area of muscle becomes infracted, the percent systolic thickening decreases (Figures 7.53, 7.54) (190,192,193). This is evident on two-dimensional and M-mode images if done carefully. The decrease in systolic thickening is not gradual from infracted areas to adjacent normal areas. It is a sudden decrease in thickening. The appearance of reduced motion toward the center of the ventricular chamber during systole on transverse or long-axis real-time images reveals infracted areas of myocardium but usually overestimates the extent of the affected area. Actual analysis of systolic thickening in the region will define the extent of the affected segment more accurately. While percent systolic thickening is an abrupt change between affected and nonaffected myocardium, wall motion abnormalities overlap between affected and adjacent areas of myocardium. These areas may display paradoxical motion and are called dyskinetic.
Myocardial Infarction
Acutely
Regional areas of decreased systolic LVW or VS thickening or systolic thinning of VS
Chronically
Fibrosis and thin areas of muscle are seen with old infarctions.
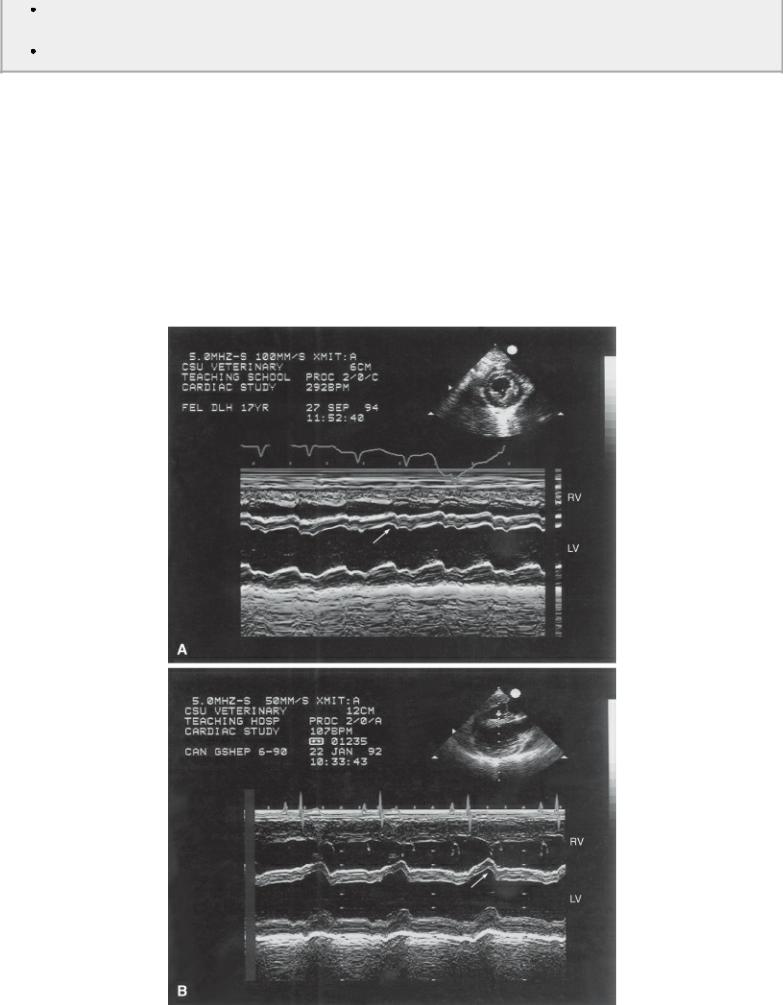
When an area of muscle displays systolic thinning it is always associated with infarcted muscle (Figure 7.55) (191,194,195). The extent of the infarction in man involves less than 20% of the myocardium when there is any evidence of systolic thickening in the segment of wall or septum being evaluated (190).
Figure 7.55 Systolic thinning of the septum or free wall on M-mode images is always associated with infarcted myocardium. (A) This cat’s M-mode shows mild systolic thinning of the septum associated with myocardial infarction. (B) The M-mode in this dog shows dramatic systolic thinning after an ischemic episode. Paradoxical septal motion secondary to right-sided volume overload would still show systolic thickening at the appropriate time. RV = right ventricle, LV = left ventricle.
